Fast Lab Solutions
CTSB enables the next step of normal life to resume: Usage of LumiraDx SARS-CoV-2 Ag Pool Test supports opening strategies for social events in Germany
J. Wintera, N. Budjina, P. Guestb, J. Ellisb
a LumiraDx GmbH, Hürth, Germany, b LumiraDx LTD, London, United Kingdom
The Corona Test Station Berlin (CTSB)
CTSB is a project initiated by several partner organisations including event managers, physicians and other key players of the German health system. Among them are the main project leaders Arne Fritsche (Executive Director CTSB Medical GmbH) and Matthias Stieler (Research & Development CTSB Medical GmbH). Fritsche and Stieler have created an outstanding test concept that utilises QR-code registration on their homepage and integrates a fully automated process using the LumiraDx SARS-CoV-2 Antigen and Antigen Pool Tests on the LumiraDx Platform, a highly sensitive test system that is able to detect 10-30 % of individuals potentially missed by SARS-CoV-2 antigen lateral flow tests1–6, and that in addition is less prone to errors as no manual interpretation of test results is required. Their fully digital test concept not only allows people to attend large events like basketball matches or film festivals by being tested for SARS-CoV-2 infection, but also enables thousands of people to be tested in a short period of time directly before the event starts.
It works by getting people to register online for a test on their homepage. In turn, the system generates a QR-code which is handed out to the customers by email. The QR-code is scanned at the testing site prior to running the test, which enables the test result to be sent securely to each customer once the test is completed. After the code is scanned, a nasal swab is collected and analysed for SARS-CoV-2 antigen. Depending on the number of waiting individuals, the samples are analysed either using the LumiraDx SARS-CoV-2 Antigen Test for individual samples or using the LumiraDx SARS-CoV-2 Antigen Pool Test, which allows for groups of up to 5 individuals to be tested on one test. This is therefore ideal for bigger events where the prevalence of COVID-19 is expected to be low, and people are required to have a recent negative antigen test result to gain entry. As part of prevention strategies, the pool test is ideal for testing groups of attendees arriving by car or charter bus directly before the event. Testing such groups of people can potentially reduce the risk of passing on infections at events, as contact with other, unfamiliar visitors before entering the event, e. g. in the queue while waiting for their test, can be prevented. CTSB has developed and delivered proof of concept of an organizational and digital means to efficiently test thousands of arriving event guests in a one-stop solution, using their event tickets combined with the scalability of LumiraDx technology and the LumiraDx SARS-CoV-2 Antigen Test. After the test is completed, people are informed about their results via email. If the result is negative, a cryptographic digital test certificate, a QR-code called BärCODE, developed and issued by Charités “Berlin Institute of Health” and/ or the national Corona Warn App cryptographic QR-code is generated and sent to the individual in the mail. Usage of these QR-codes avoids false certificates that might cause multi-spreading events and can be scanned directly when entering any event. If a SARS-CoV-2 Ag Pool Test result is positive, the individual receives the notification of a positive pool test via email and is requested to attend for retesting with a SARS-CoV-2 antigen individual test. In addition, a positive test result leads to blacklisting tickets until retesting shows a negative result. If the subsequent test is negative people are allowed to enter the event, if the individual test is positive, the affected individual is requested to isolate at home and verification of the test result with a PCR test is required. In parallel, the local health system will be informed automatically by the digital infrastructure implemented in the test concept. This institution organises the retesting with PCR to verify the antigen test result, as well as contact tracing and isolation in case of a SARS-CoV-2 positive PCR result.
The LumiraDx SARS-CoV-2 Ag Pool Test
The LumiraDx SARS-CoV-2 Ag Pool Test is a rapid microfluidic immunofluorescence assay for the qualitative detection of the nucleocapsid protein antigen in nasal or nasopharyngeal swab specimens pooled from up to 5 individuals suspected of COVID-19 or up to 5 asymptomatic individuals. Used with the LumiraDx Platform, the test offers a rapid, scalable, and cost-effective screening solution for potentially infectious individuals, that in addition offers a connectivity tool for direct transfer of test results to a compatible software solution.
CTSB tested over 10000 people with the LumiraDx SARS-CoV-2 Ag Pool Test in 2 months
In May and June 2021, CTSB tested 24180 people for SARS-CoV-2 antigen with products from LumiraDx to allow entry to several social events such as the ALBA basketball matches, soccer matches such as the DFB finals, or the Berlinale Film Festival. Among them, 10543 individuals were tested with the LumiraDx SARS-CoV-2 Antigen Pool Test in 2836 pools. Of these, 15 pools were positive and consequently required re-testing of 54 individuals. Due to the current valid data protection guidelines, it was unfortunately not possible to determine whether the individuals who were previously tested positive by pools could also be verified in the individual antigen tests (but observationally, at least one positive was confirmed per pool by individual testing). With this taken into account, it is not possible to reproduce the exact number of SARS-CoV-2 Ag positive individuals that have been analysed with the pool test, therefore calculation of exact prevalence was not possible, however the prevalence was estimated in both groups according to the range of potential numbers of positives. However, the prevalence range estimated for the single test 0.20-0.30 % (95 % CI* 0.14- 0.41 %) overlaps with the estimated prevalence for pooled tests 0.14-0.51 % (95 % CI 0.09-0.67 %), therefore, the prevalence is comparable for both products.
*CI: Confidence Interval
Estimated specificity of the LumiraDx SARS-CoV-2 Antigen Pool Test is excellent
In the observed setting, the estimated specificity for the LumiraDx SARS-CoV-2 Antigen and Antigen Pool Test was excellent. For both tests, a very low number of positives was observed. Even if all of those samples would be classified as false positives, the resulting negative percent agreement (NPA) would be even higher than that stated by the manufacturer (NPApool = 99.5 %, NPAsingle = 99.7 %, NPAmanufacturer = 98.8 %).
The LumiraDx Platform in combination with the LumiraDx SARS-CoV-2 Antigen and Antigen Pool Test is easy to use and results in a very low error rate
In accordance with the current German testing strategy, non-healthcare professionals were trained on how to correctly collect swabs from individuals and how to perform the test. After this, they were considered to be trained staff as per the LumiraDx Product Insert and were therefore allowed to use IVDs with CE for professional use, such as the LumiraDx Platform and related tests. The platform was considered as easy to use by the staff because over all measurements only 409 errors occurred. This reflects a total error rate of only 2.42 % (Table 1). Further, the error rate was slightly higher in single tests (2.46 %) compared to pool tests (2.24 %) but did not reach significance. The platform was also deemed to be very intuitive by staff.
| Total | Single test | Pooled test | |
|---|---|---|---|
| Number of errors | 409 | 344 | 65 |
| Number of runs (n test strips valid measures) | 16473 | 13637 | 2836 |
| Number of runs (valid measures + errors) | 16882 | 13981 | 2901 |
| Error rate in % | 2.42 | 2.46 | 2.24 |
Table 1: Error rate detected over 2 months at the CTSB.
A more detailed look on the underlying error codes revealed that 63 % of all errors were based on handling errors and 37 % were caused by technical issues (strip or instrument error) (Figure 1). In summary, the overall error rate of 2.4 % can be further divided into 0.9 % errors caused by technical reasons and 1.5 % for handling issues only. The most common user error was timeout that occurred after sample application, where the door of the device was not closed within the required 10 seconds after sample detection.
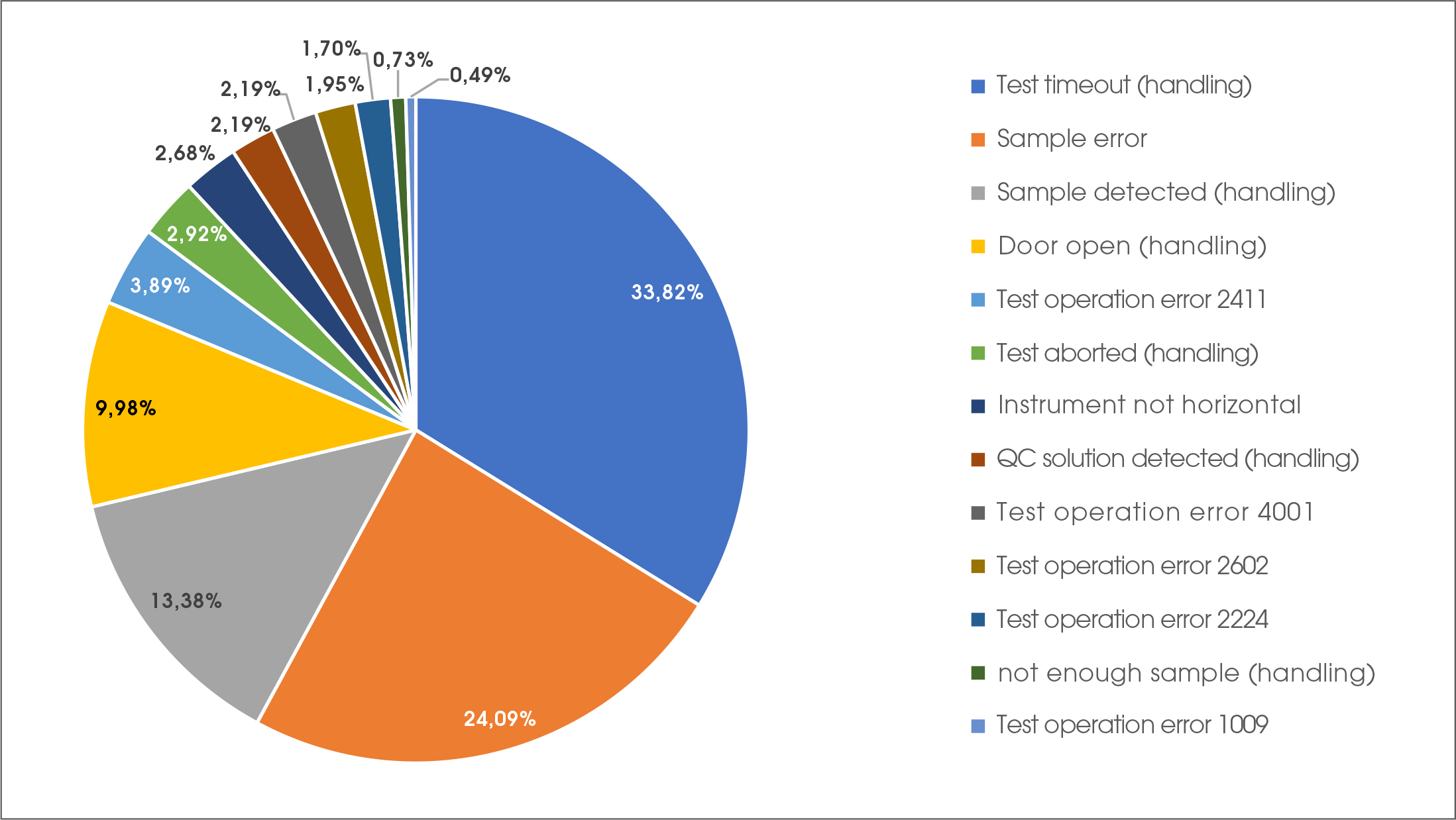
Figure 1: Different errors which occurred over 2 months observation time. Results arepresented as % of total errors based on different error codes produced by the device.
Use of LumiraDx SARS-CoV-2 Antigen Pool Test saved time and costs
Based on the numbers of used test strips, tested individuals, and the currently applicable pricing in Germany, the use of the LumiraDx SARS-CoV-2 Antigen Pool Test saved 65.4 % of costs for test strips which corresponds to 123107 €, when compared to testing all individuals using the LumiraDx SARS-CoV-2 individual tests.
In addition, as the individual testing takes more time than the pool testing, calculations revealed that 191 additional working days would have been required if all individuals who were tested by pool tests had been tested with individual tests instead. This represents additional workforce/staffing of 4.3 employees based on the duration of the observation of 2 months, further increasing the overall cost of the operation.
Conclusion
The LumiraDx SARS-CoV-2 Antigen Pool Test is an easy to use, efficient, and cost & time saving SARS-CoV-2 Antigen Test that demonstrated outstanding usability and utility in a real life setting with more than 10000 individuals tested. The observed low error rate, good performance, and intuitive, easy handling, shows it represents a useful tool for screening settings such as access control at social events where a high number of individuals need to be tested in a short time frame, and when a low prevalence of COVID-19 is expected.
References:
1. Osterman, A.; Baldauf, H.-M.; Eletreby, M.; Wettengel, J. M.; Afridi, S. Q.; Fuchs, T.; Holzmann, E.; Maier, A.; Döring, J.; Grzimek-Koschewa, N.; Muenchhoff, M.; Protzer, U.; Kaderali, L.; Keppler, O. T. Evaluation of Two Rapid Antigen Tests to Detect SARS-CoV-2 in a Hospital Setting. Med. Microbiol. Immunol. (Berl.) 2021, 210 (1), 65–72. https://doi.org/10.1007/s00430-020-00698-8.
2. Young, S.; Taylor, S. N.; Cammarata, C. L.; Varnado, K. G.; Roger-Dalbert, C.; Montano, A.; Griego-Fullbright, C.; Burgard, C.; Fernandez, C.; Eckert, K.; Andrews, J. C.; Ren, H.; Allen, J.; Ackerman, R.; Cooper, C. K. Clinical Evaluation of BD Veritor SARS-CoV-2 Point-of-Care Test Performance Compared to PCR-Based Testing and versus the Sofia 2 SARS Antigen Point-of-Care Test. J Clin Microbiol 2020, 59 (1). https://doi.org/10.1128/jcm.02338-20.
3. Mak, G. C. K.; Lau, S. S. Y.; Wong, K. K. Y.; Chow, N. L. S.; Lau, C. S.; Lam, E. T. K.; Chan, R. C. W.; Tsang, D. N. C. Evaluation of Rapid Antigen Detection Kit from the WHO Emergency Use List for Detecting SARS-CoV-2. J. Clin. Virol. 2021, 134, 104712. https://doi.org/10.1016/j.jcv.2020.104712.
4. Linares, M.; Pérez-Tanoira, R.; Carrero, A.; Romanyk, J.; Pérez-García, F.; Gómez-Herruz, P.; Arroyo, T.; Cuadros, J. Panbio Antigen Rapid Test Is Reliable to Diagnose SARS-CoV-2 Infection in the First 7 Days after the Onset of Symptoms. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2020, 133, 104659–104659.https://doi.org/10.1016/j.jcv.2020.104659.
5. Drain, P. K.; Ampajwala, M.; Chappel, C.; Gvozden, A. B.; Hoppers, M.; Wang, M.; Rosen, R.; Young, S.; Zissman, E.; Montano, M. A Rapid, High-Sensitivity SARS-CoV-2 Nucleocapsid Immunoassay to Aid Diagnosis of Acute COVID-19 at the Point of Care: A Clinical Performance Study. Infect. Dis. Ther. 2021, 10 (2), 753–761. https://doi.org/10.1007/s40121-021-00413-x.
6. Brümmer, L. E.; Katzenschlager, S.; Gaeddert, M.; Erdmann, C.; Schmitz, S.; Bota, M.; Grilli, M.; Larmann, J.; Weigand, M. A.; Pollock, N. R.; Macé, A.; Carmona, S.; Ongarello, S.; Sacks, J. A.; Denkinger, C. M. Accuracy of Novel Antigen Rapid Diagnostics for SARS-CoV-2: A Living Systematic Review and Meta-Analysis. PLOS Med. 2021, 18 (8), e1003735. https://doi.org/10.1371/journal.pmed.1003735.
This document was produced by LumiraDx UK Ltd. Copyright © 2021 LumiraDx UK LTD. All rights reserved, worldwide.
Not available in all countries and regions. Please check with your local LumiraDx sales representative or distributor for availability in specific markets. Not available in the USA.
BetterHealthExperiencesOutcomes
Supporting healthier lives, for individuals, communities and wider society.
Enabling responsive, personal relationships between patients and care teams.
Controlling and reducing costs to help ease pressure on healthcare budgets.