HbA1c Test
an easy to use, fast microfluidic immunoassay designed to rapidly quantify HbA1c in fingerstick and venous whole blood. Used with the LumiraDx Platform, the LumiraDx HbA1c test delivers rapid, reliable results in <5 minutes at the point of care.
Quantitative HbA1c test
The LumiraDx HbA1c test is an in vitro diagnostic test for the quantitative determination of haemoglobin A1c in human capillary and venous whole blood samples.
Near-patient testing
A fully automated in vitro diagnostic test, designed for community-based settings, where the patient presents, for increased efficiency and improved patient experience.
Purpose
The test can be used for the monitoring of long-term glycaemic control in individuals with diabetes mellitus, and as an aid in screening and identifying patients who may be at risk for developing diabetes.
Benefits

Actionable, lab-comparable results from a single fingerstick sample
Monitor known diabetic patients’ HbA1c levels at the point of need and improve efficiency with on-the-spot diabetes screening at the patient’s side:
- NGSP Certified
- IFCC traceable
- Sample types: fingerstick or venous whole blood (EDTA), via lysis device
- Sample size: 15 μL
- Measuring range: 20 - 130 mmol/mol (4.0 –14.0%)
- Time to result: <5 minutes
- Storage at room temperature (2°C to 30°C)

Workflow
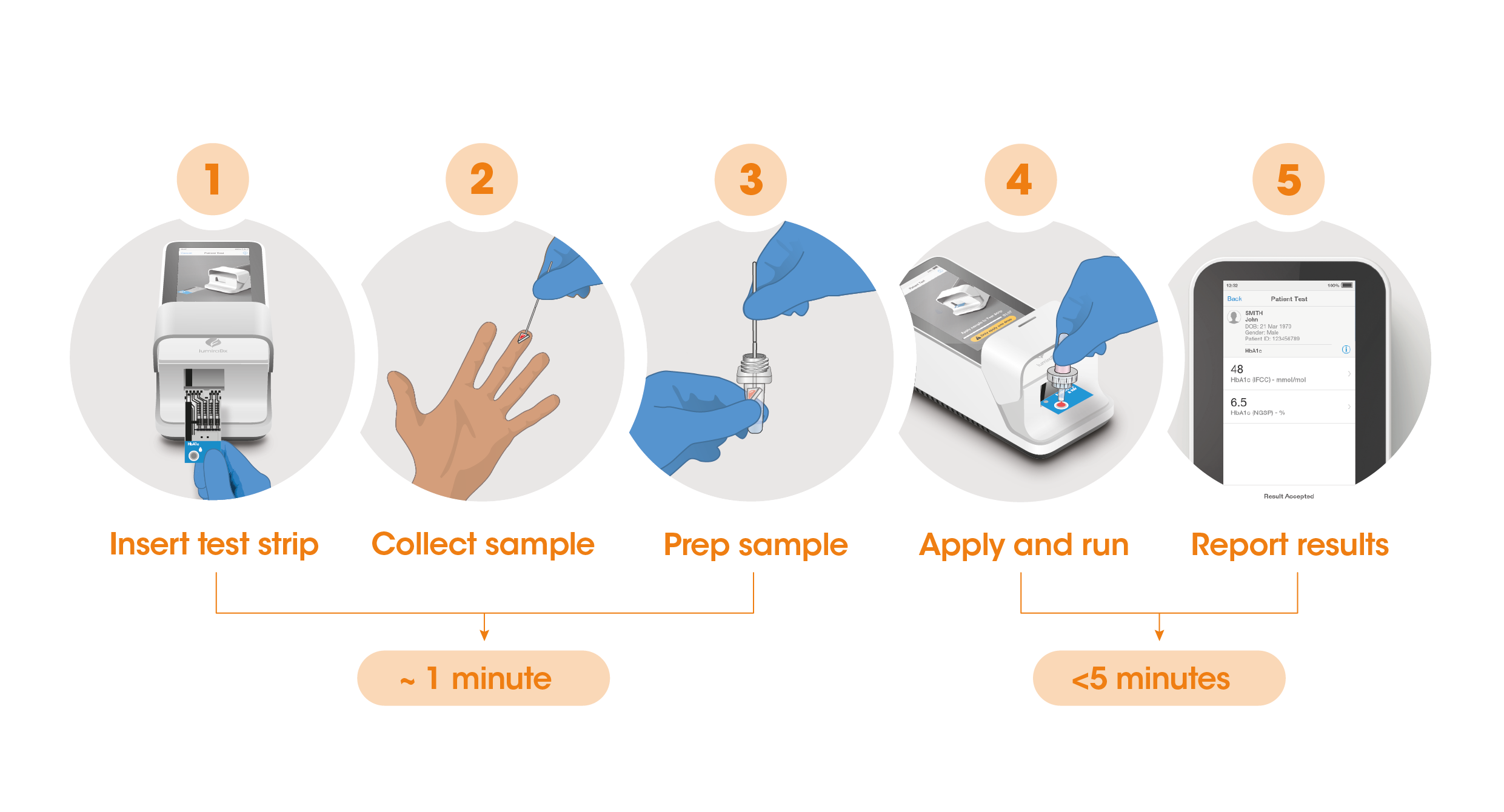
Test results in 5 minutes
The workflow process is comprised of a fingerstick sample collection, followed by step-by-step guidance of the Instrument to report a patient result in <5 minutes from sample application.
The Instrument and test strips are integrated with several quality control checks to ensure the Instrument and test are functioning correctly for every test run.
HOW TO USE VIDEOTest Performance
Method comparison
The LumiraDx HbA1c test was evaluated at the European Reference Laboratory for Glycohaemoglobin (ERL) by Dr. Erna Lenters-Westra (Clinical Chemistry Department, Isala, Zwolle, The Netherlands). The aim of the evaluation was to assess the performance of fresh EDTA whole blood samples compared to values assigned using four IFCC and NGSP certified Secondary Reference Measurement Procedures (SRMPs)*. The method comparison results between the mean of the 4 SRMPs and the mean of the 2 LumiraDx Instruments can be found by clicking above.
*The 4 IFCC and NGSP SRMPs used in this evaluation were:
1. Roche Tina-quant Gen.3 HbA1c on Cobas c513, immunoassay, IFCC and NGSP certified SRMP
(Roche Diagnostics)
2. Premier Hb9210, boronate affinity chromatography HPLC, IFCC and NGSP certified SRMP (Trinity
Biotech)
3. Tosoh G11, cation-exchange HPLC, IFCC certified SRMP (Tosoh Bioscience)
4. Abbott Enzymatic method on Alinity, IFCC and NGSP certified SRMP (Abbott Diagnostics).
Test precision
A precision study was carried out in venous whole blood (EDTA) on a protocol based on CLSI EP5-A3. The study was carried out at 2 concentrations of HbA1c, each was tested in 1 run of 5 replicates per day, for 5 days across 3 sites. The results of the precision study were 4.0% and 3.4% CV across the two levels. Click above for more details.
Product Documentation
HbA1c Test:
HbA1c Test

