SARS-CoV-2
Ag Pool Test
The LumiraDx SARS-CoV-2 Ag Pool Test is a rapid microfluidic immunofluorescence assay for the qualitative detection of the nucleocapsid protein antigen in nasal or nasopharyngeal swab specimens pooled from up to 5 individuals suspected of COVID-19 or up to 5 asymptomatic individuals. Used with the LumiraDx Platform the test offers a rapid, scalable and cost-effective screening solution for infectious individuals.
Test benefits
- Quickly and easily scale up testing: Low-cost diagnostic screening solutions
- Maximize resources: Pooled testing can improve access to testing by significantly reducing resources (tests, operators, instruments) required on a per patient basis
- Save Time: Pooled testing can result in a 40-60% reduction in testing, allowing for significant savings in both time and cost
- High sensitivity of up to Ct 33 enables the accurate detection of the majority of infectious patients
- Easy to implement in point of care settings
- Room Temperature test strip storage
- Time to result in 12 minutes
- Compact and portable instrument with connectivity options
- Facilitate reopening of work sites, sports, cultural venues and events and travel
- Regular testing of defined populations e.g. healthcare staff, healthcare facility residents, employees, students
- One time testing of groups for pre entry or reopening of sites
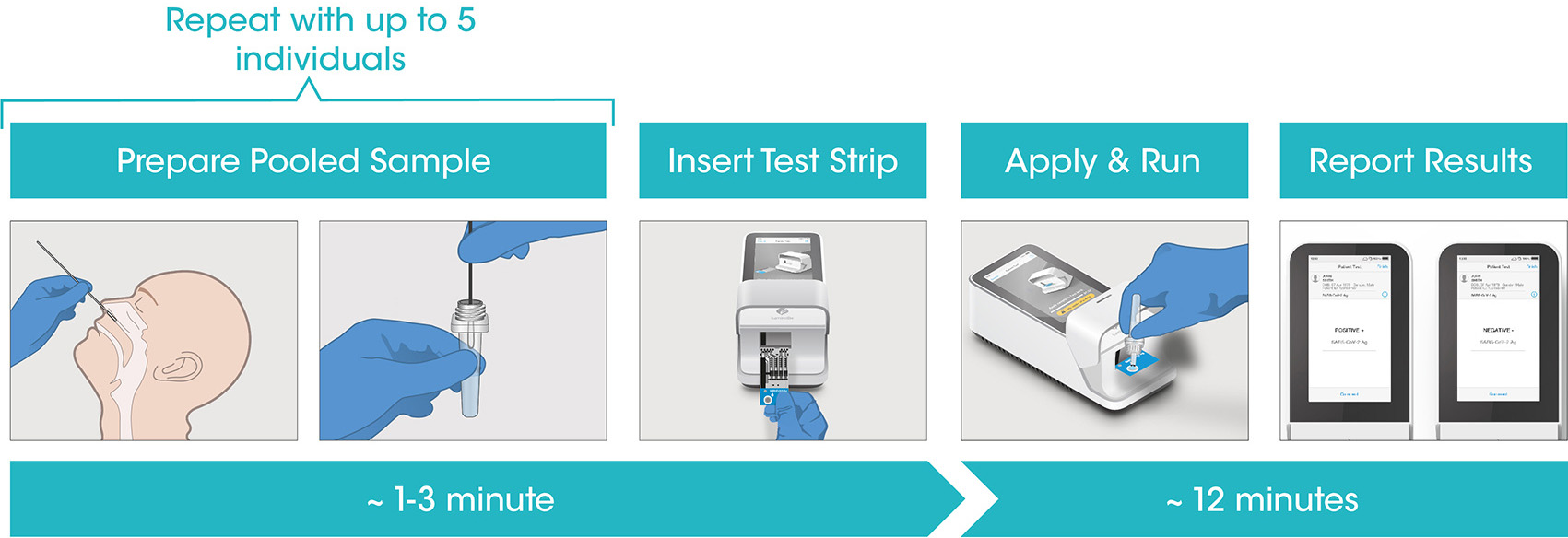
Test workflow
The Instrument and Test Strips are integrated with several quality control checks to ensure the Instrument and Test are functioning correctly for every test run.
The workflow process is comprised of a simple sample prep along with step-by-step guidance of the Instrument to report a patient result in under 12 minutes from sample application.
Test performance
In clinical studies, the LumiraDx SARS-CoV-2 antigen pool test demonstrated 100% positive agreement and 96.6% negative agreement when compared to testing individual swabs on the LumiraDx SARS-COV-2 Ag Test, thereby ensuring high sensitivity is maintained while reducing overall testing times, costs and operators required for testing.
| Measure | Estimate | 95 % Wilson Score CI | |
|---|---|---|---|
| PPA | 100.0 % | 88.6 % | 100.0 % |
| NPA | 96.6 % | 82.8 % | 99.4 % |
| PPV | 96.8 % | 83.8 % | 99.4 % |
| NPV | 100.0 % | 87.9 % | 100.0 % |
Limit of detection
LOD results when conducted with SARS-CoV-2 Pool Test (1 positive swab and 4 negative swabs) were comparable to LOD studies run with the SARS-CoV-2 Ag Test, which has an LOD of 32 TCID50/mL
Test performance in asymptomatic population
The performance of the LumiraDx SARS-CoV-2 Ag Test was established with direct nasal and nasopharyngeal swabs prospectively collected from asymptomatic individuals during the 2020 pandemic.
| Grouping | Nasal swabs | NP swabs | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | PPA | 95% CI | NPA | 95% CI | n | PPA | 95% CI | NPA | 95% CI | |
| Asymptomatic | 309 | 82.4% | 59.0-93.8% | 99.3% | 97.5-99.8% | 192 | 80.0% | 49.0-94.3% | 98.4% | 95.3-99.4% |
| Ct <33 | 13 | 100% | 77.2-100% | - | - | 7 | 100% | 64.6-100% | - | - |
For more information about the LumiraDx SARS-CoV-2 Ag Pool Test
Not all products are available in all countries and regions. Please check with your local LumiraDx sales representative or distributor for availability in specific markets. Not available in the USA.
BetterHealthExperiencesOutcomes
Supporting healthier lives, for individuals, communities and wider society
Enabling responsive, personal relationships between patients and care teams.
Controlling and reducing costs to help ease pressure on healthcare budgets.