HbA1c Test
The LumiraDx HbA1c test is an easy to use, fast microfluidic immunoassay designed to rapidly quantify HbA1c in fingerstick and venous whole blood. Used with the LumiraDx Platform, the LumiraDx HbA1c test delivers rapid, reliable results in < 5 minutes at the point of care.
The LumiraDx HbA1c test is an in vitro diagnostic test for the quantitative determination of haemoglobin A1c (IFCC mmol/mol and NGSP %) in human capillary and venous whole blood samples (EDTA). The LumiraDx HbA1c Test Strips are intended for use with the LumiraDx Instrument. It is an automated in vitro diagnostic test for near-patient testing.
HbA1c is used for the monitoring of long-term glycaemic control in individuals with diabetes mellitus, and as an aid in screening and identifying patients who may be at risk for developing diabetes. The LumiraDx HbA1c test is for Professional Use Only. For patients ≥2 years of age.
Test benefits
The measurement of HbA1c allows physicians and pharmacists to monitor patients with diabetes, or as an aid to screen and identify those at risk for developing diabetes.
- Certification: IFCC, NGSP
- Sample types: capillary fingerstick or venous whole blood (EDTA) via lysis device
- Sample size: 15μL
- Time to result: < 5 minutes
- Precision (%CV): Approx. 2% NGSP, 3% IFCC (single instrument)
- Measuring range: 20 - 130 mmol/mol (4.0 – 14.0%)
- No significant Hb variant interference from HbS, HbC, HbD or HbE*
- Storage at room temperature
The LumiraDx HbA1c test is a smart, automated, highly portable solution designed to improve access and ease of use in community-based healthcare settings.
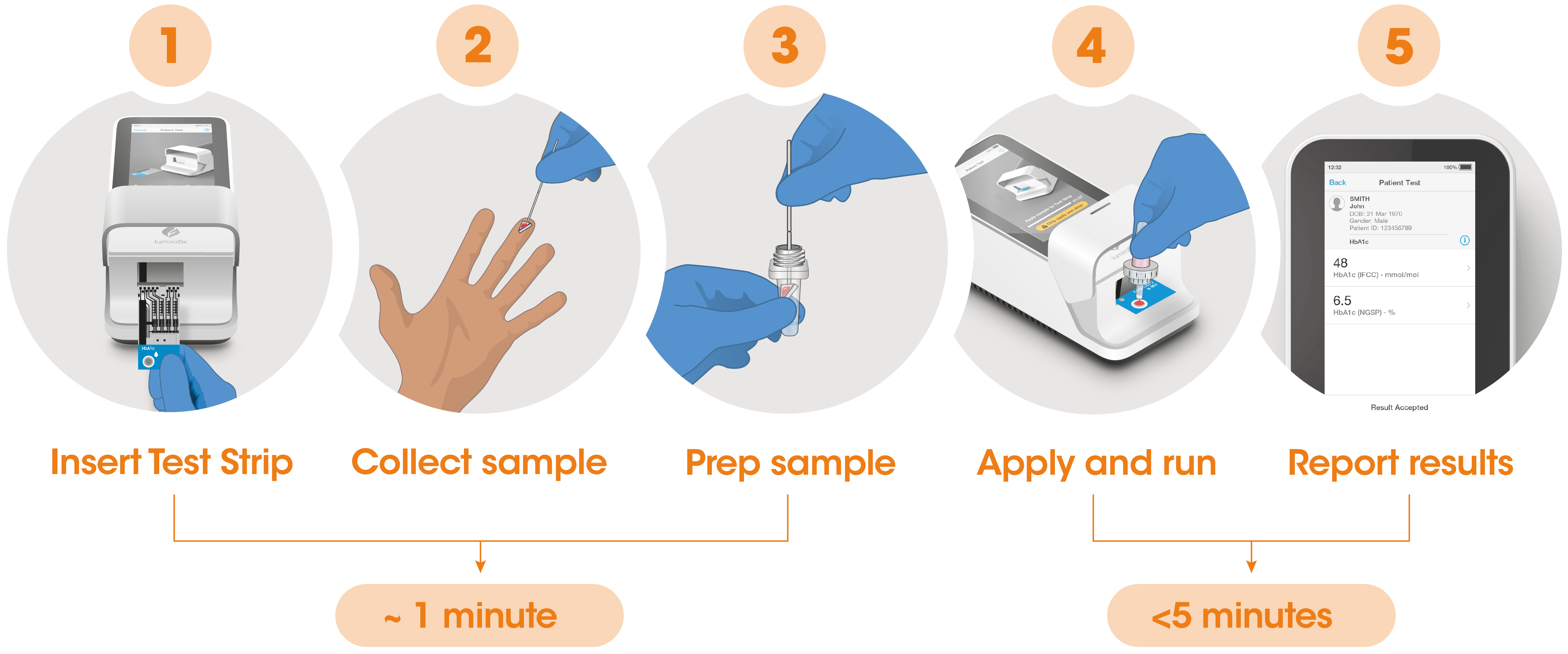
Test workflow
The Instrument and Test Strips are integrated with several quality control checks to ensure the Instrument and Test are functioning correctly for every test run.
The workflow process is comprised of a simple sample collection with a fingerstick lancet followed by step-by-step guidance of the Instrument to report a patient result in <5 minutes from sample application.
Test performance
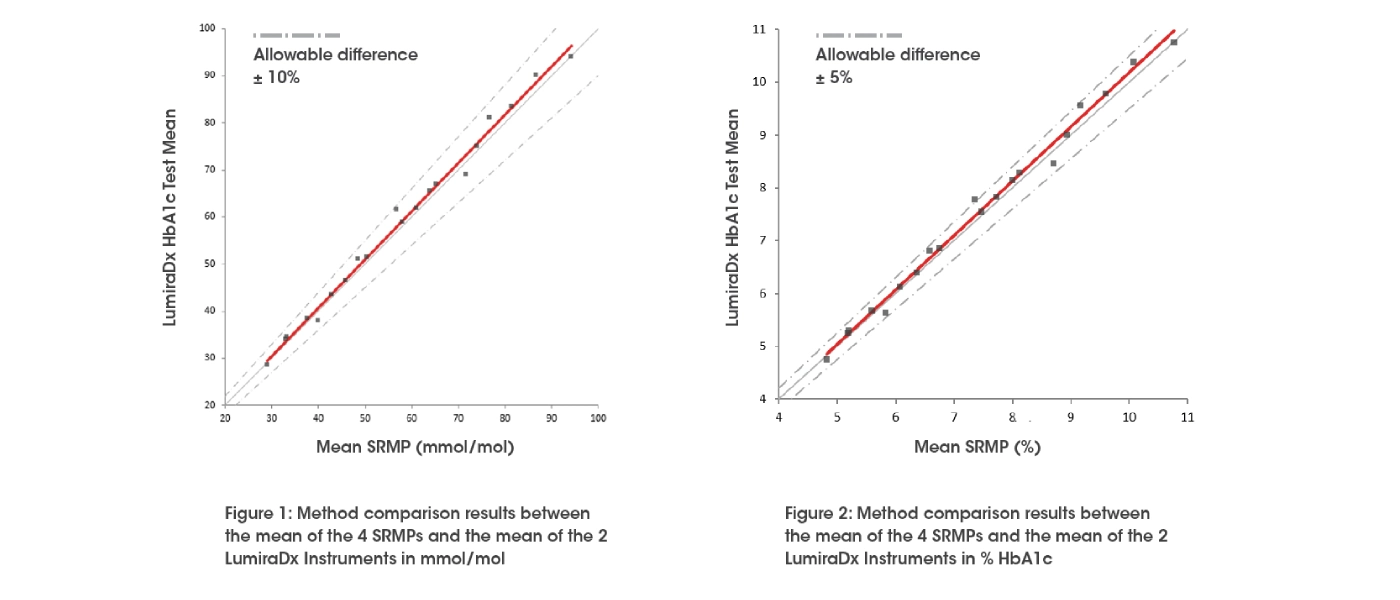
Method comparison
The LumiraDx HbA1c Test was evaluated at the European Reference Laboratory for Glycohaemoglobin (ERL) by Dr. Erna Lenters-Westra (Clinical Chemistry Department, Isala, Zwolle, The Netherlands). The aim of the evaluation was to assess the performance of fresh EDTA whole blood samples compared to values assigned using four IFCC and NGSP certified Secondary Reference Measurement Procedures (SRMPs)**. The method comparison results between the mean of the 4 SRMPs and the mean of the 2 LumiraDx Instruments can be found below.
* See LumiraDx HbA1c Test Product Insert for additional details
More information about the LumiraDx HbA1c Test:
Not all products are available in all countries and regions. Please check with your local LumiraDx sales representative or distributor for availability in specific markets.
BetterHealthExperiencesOutcomes
Supporting healthier lives, for individuals, communities and wider society
Enabling responsive, personal relationships between patients and care teams.
Controlling and reducing costs to help ease pressure on healthcare budgets.