Fast Lab Solutions
An evaluation of the detection of SARS-CoV-2 antigen in a cohort of symptomatic and asymptomatic patients: Report from the evaluation of SKUP

Aim
The aim of the evaluation was to assess the diagnostic performance and user-friendliness of LumiraDx SARSCoV-2 Ag Test when used under real life conditions by intended users in a dedicated COVID-19 testing centre with symptomatic and asymptomatic subjects.
Materials and methods
One nasal and two nasopharyngeal swab samples were taken at the same time from 450 subjects at Bergen Accident and Emergency Clinic. The subjects, 16 years or older, had been exposed to an individual who had previously tested positive for SARS-CoV-2. The nasal swab and one of the nasopharyngeal swabs were used for measurement with LumiraDx SARS-CoV-2 Ag Test, and the other nasopharyngeal swab was sent to the clinical microbiology laboratory at Haukeland University Hospital for measurement on an in-house RT-PCR comparison method. Userfriendliness was assessed using a questionnaire with three ratings; satisfactory, intermediate and unsatisfactory, and with the quality goal of a total rating of “satisfactory”.
Results
The prevalence of SARS-CoV-2 among the participants was 18.5 %. Of those recruited, 448 were included in the final analysis with complete datasets. Of these, 56 % were symptomatic. Amongst the symptomatic group, 87 % presented with symptoms ≤5 days since onset. Demographic characteristics are presented in Table 1.
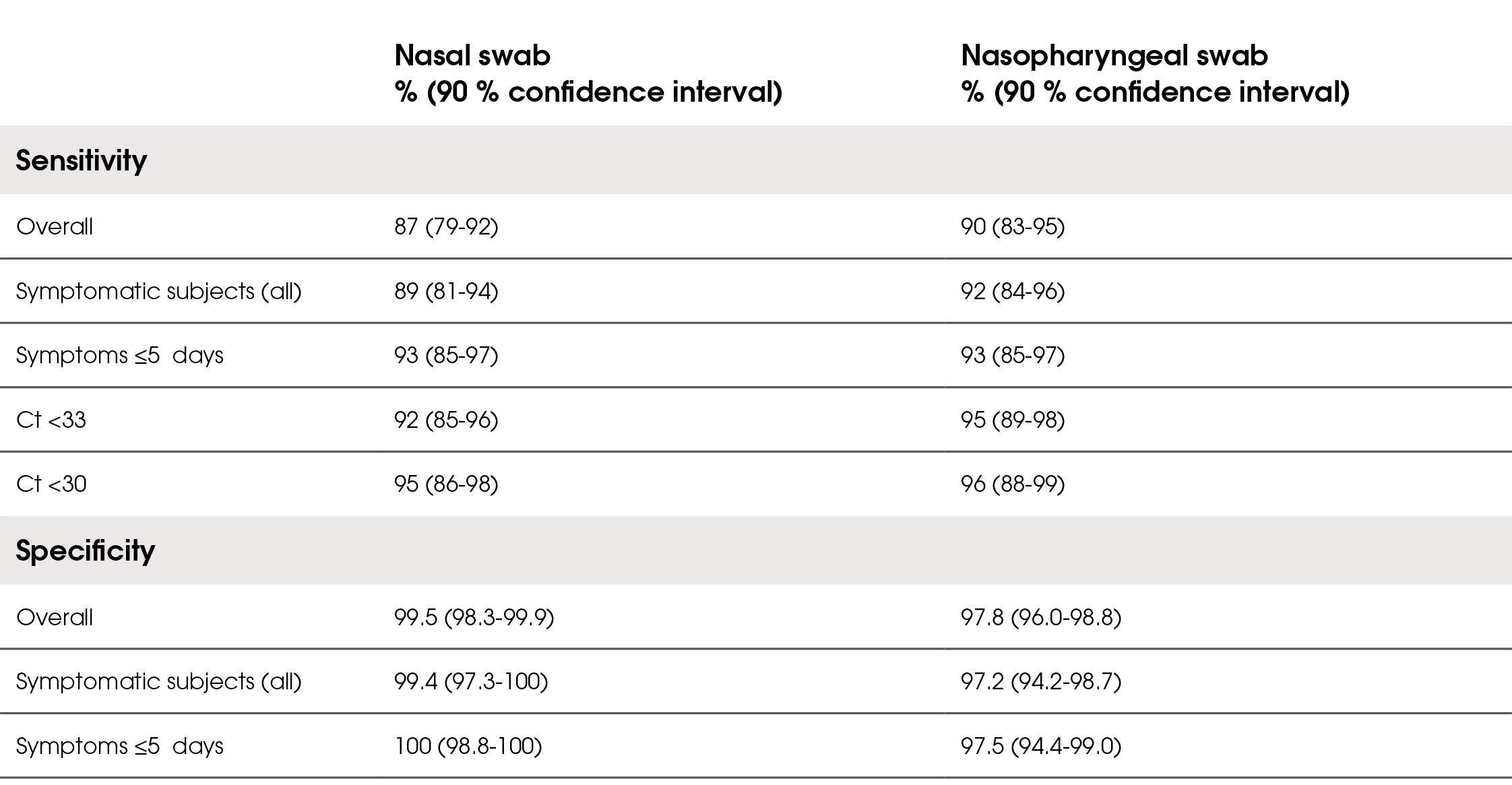
The overall diagnostic sensitivity of LumiraDx SARSCoV-2 Ag Test in the symptomatic and asymptomatic population was 87 % for the nasal samples and 90 % for the nasopharyngeal samples. Of those presenting with symptoms, sensitivity was 89 % for the nasal swab and 92 % for the nasopharyngeal samples. In symptomatic subjects presenting within 5 days of symptom onset, sensitivity was 93 % in both sample types. Most of the false negative results had ct values ≥33, resulting in sensitivity for nasal sample of 92 % and 95 % for the nasopharyngeal sample. The overall diagnostic specificity was 99.5 % for the nasal samples and 97.8 % for the nasopharyngeal samples. The positive predictive values of the test were 97 % for the nasal samples and 90 % for the nasopharyngeal samples. The negative predictive values of the test were 97.1 % for the nasal samples and 97.8 % for the nasopharyngeal samples. The user-friendliness was rated as satisfactory. Results are summarised in Table 2.
Table 1. Population characteristics of included subjects
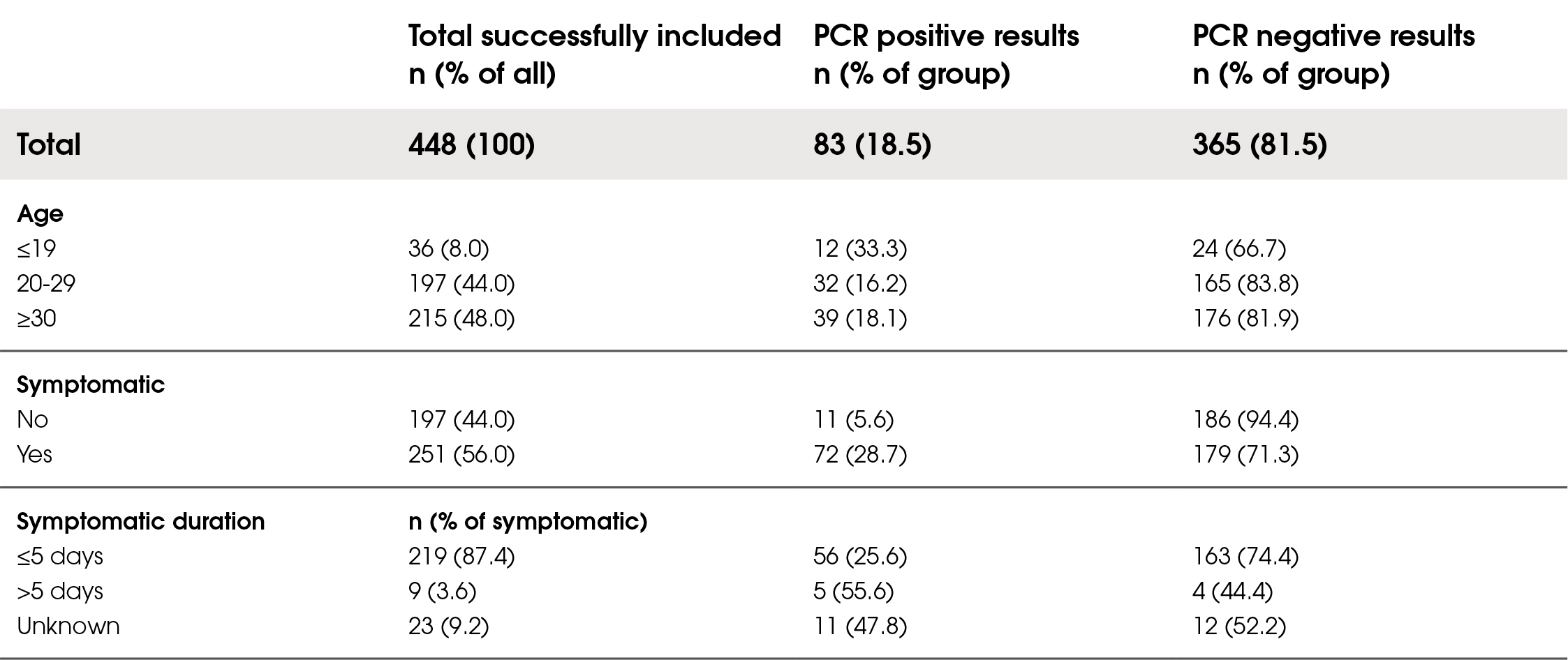
Table 2. Diagnostic sensitivity and specificity of the LumiraDx SARS-CoV-2 Ag Test
Conclusion
LumiraDx SARS-CoV-2 Ag Test fulfils the WHO minimum performance requirement for diagnostic sensitivity (≥80 %) and specificity (≥97 %),1 when used under real-life conditions, with a prevalence of 18.5 % by the intended users
Report SKUP/2020/124
SKUP: Scandinavian evaluation of laboratory equipment for point of care testing, SKUP, is a co-operative commitment of DEKS in Denmark, Noklus in Norway and Equalis in Sweden. SKUP was established in 1997 at the initiative of laboratory medicine professionals in the three countries. SKUP is led by a Scandinavian steering committee and the secretariat is located at Noklus in Bergen, Norway.
1. WHO www.who.int “COVID-19 Target product profiles for priority diagnostics to support response to the COVID-19 pandemic” v.1.0 Accessed Jan 2021
BetterHealthExperiencesOutcomes
Supporting healthier lives, for individuals, communities and wider society.
Enabling responsive, personal relationships between patients and care teams.
Controlling and reducing costs to help ease pressure on healthcare budgets.