Fast Lab Solutions
SARS-CoV-2
Ag Ultra Test
Lab-comparable results in just 5 minutes.
With excellent performance and results in just 5 minutes, the LumiraDx SARS-CoV-2 Ag Ultra test enables you to accurately and confidently test more patients, optimize clinic workflows and help triage patients without delay.
Test benefits
- Microfluidic immunofluorescence assay
- Results in 5 minutes
- Optimize clinical workflow
- Sample type: nasal swab
- Easy to implement at point of care
- No refrigeration storage required: room temperature storage (2-30°C)
- Small and portable Instrument
- Minimal handling steps

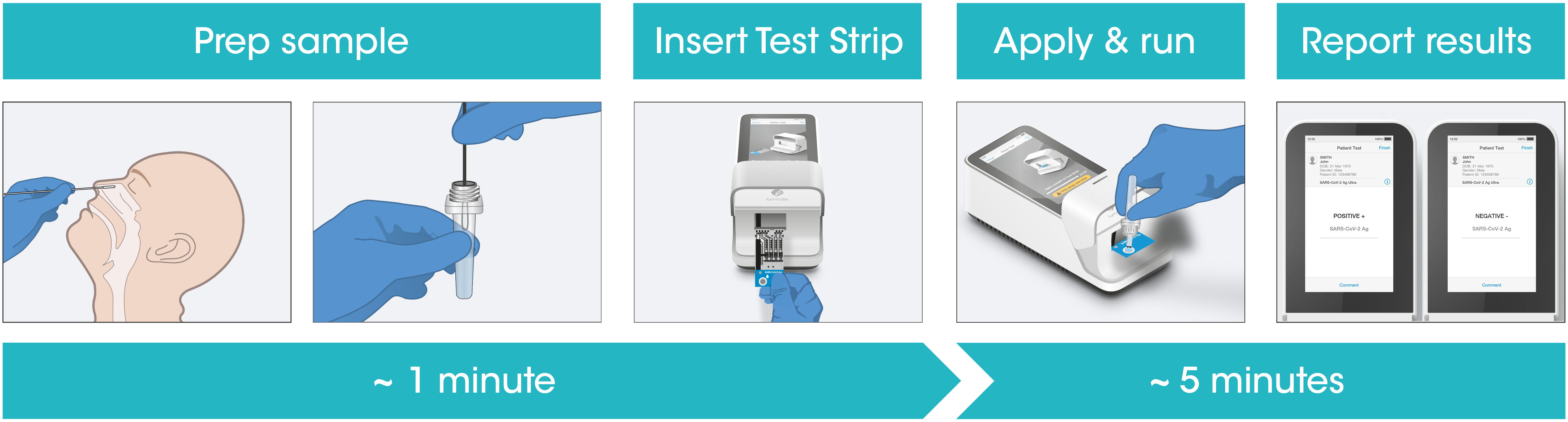
Test workflow
The Instrument and Test Strips are integrated with several quality control checks to ensure the Instrument and Test are functioning correctly for every test run.
The workflow process is comprised of a simple sample prep along with step-by-step guidance of the Instrument to report a patient result in under 5 minutes from sample application.
Test performance
In clinical studies, the LumiraDx SARS-CoV-2 Ag Ultra test demonstrated 97.4%* positive agreement versus RT-PCR from individuals with symptoms.
| SARS-CoV-2 | |
|---|---|
| PPA* | 97.4% |
| NPA | 100% |
*Ct <34
See LumiraDx SARS-CoV-2 Ag Ultra Pool Product Insert for more details
High sensitivity in patients with a Ct value <34 within 12 days of the onset of symptoms – Rapidly identify potentially contagious spreaders.
The following table shows the performance grouped by Ct value, illustrating that the LumiraDx SARS-CoV-2 Ag Ultra test has high sensitivity in patients with a Ct value <34.
Samples with Cts above 33-34 are generally considered to be non-infectious1 indicating that the LumiraDx SARS-CoV-2 Ag Ultra test can rapidly identify potentially contagious spreaders.
| Grouping | N | PPA |
|---|---|---|
| Ct (all) | 41 | 92.7% |
| Ct < 34 (all) | 39 | 97.4% |
| Ct < 33 (all) | 38 | 97.4% |
| Ct < 30 (all) | 35 | 97.1% |
| Ct < 25 (all) | 25 | 100.0% |
High sensitivity for detection of SARS-CoV-2 in asymptomatic individuals
In clinical studies, the LumiraDx SARS-CoV-2 Ag Ultra test demonstrated 95.7% positive agreement versus RT-PCR in samples collected from individuals without symptoms or other epidemiological reasons to suspect COVID-19.
With the Omicron variant showing a much higher rate of asymptomatic carriage compared to other variants2, the high sensitivity of the LumiraDx SARS-CoV-2 Ag Ultra Test can be an important tool in breaking the chain of transmission.
| SARS-CoV-2 | |
|---|---|
| PPA | 95.7% |
| NPA | 100% |
See LumiraDx SARS-CoV-2 Ag Ultra Product Insert for more details
1. La Scola B., Le Bideau M., Andreani J., Hoang V.T., Grimaldier C., Colson P. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur J Clin Microbiol Infect Dis. 2020:1–3.
2. Garrett N, Tapley A, Andriesen J, et al. High Rate of Asymptomatic Carriage Associated with Variant Strain Omicron. Preprint. medRxiv. 2022;2021.12.20.21268130. Published 2022 Jan 14. doi:10.1101/2021.12.20.21268130
More info about the LumiraDx SARS-CoV-2 Ag Ultra Test:
Not all products are available in all countries and regions. Please check with your local LumiraDx sales representative or distributor for availability in specific markets.
BetterHealthExperiencesOutcomes
Supporting healthier lives, for individuals, communities and wider society.
Enabling responsive, personal relationships between patients and care teams.
Controlling and reducing costs to help ease pressure on healthcare budgets.