Fast Lab Solutions
INR Test
The LumiraDx INR Test measures prothrombin time reported as International Normalized Ratio (INR) from a single, direct fingerstick blood sample – all in less than 90 seconds*. The Test is used for monitoring patients on oral anticoagulation therapy with Vitamin-K Antagonist (VKA) drugs. Used with the LumiraDx Instrument, the Test delivers rapid results at the point of care.
Test benefits
INR is the preferred test for monitoring patients on vitamin K antagonist (VKA) therapy. The current standard method for monitoring INR is laboratory testing. However, the LumiraDx INR Test is performed at the point of care, which provides several benefits over laboratory testing.
- Improved convenience – on the spot testing – at the patient’s side
- Faster turnaround times when compared to laboratory testing – results in less than 90 seconds*
- Faster diagnosis and management of treatment leads to improved clinical outcomes
- Reduced number of referrals – reducing the burden upon secondary care
- Precise & accurate – CVs <5% and correlation with the Instrumentation Laboratory ACL Elite laboratory reference method
- Reportable range: 0.8 - 8.0 INR**
- Room temperature Test Strip storage
* Supratherapeutic INR > 4.0 test result could take 90-180 seconds
**Applicable from INR Test Strip Lot 5000811 onwards
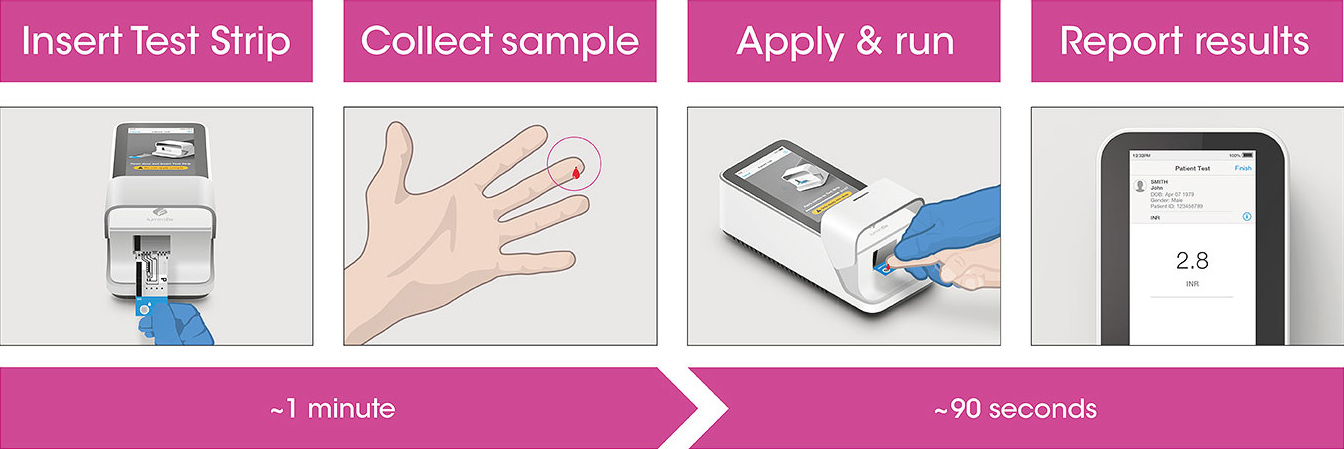
Test workflow
The Instrument and Test Strips are integrated with several quality control checks to ensure the Instrument and Test are functioning correctly for every test run.
The workflow process is comprised of a single fingerstick and direct application to the Test Strip, along with step-by-step guidance of the Instrument to report a patient result.
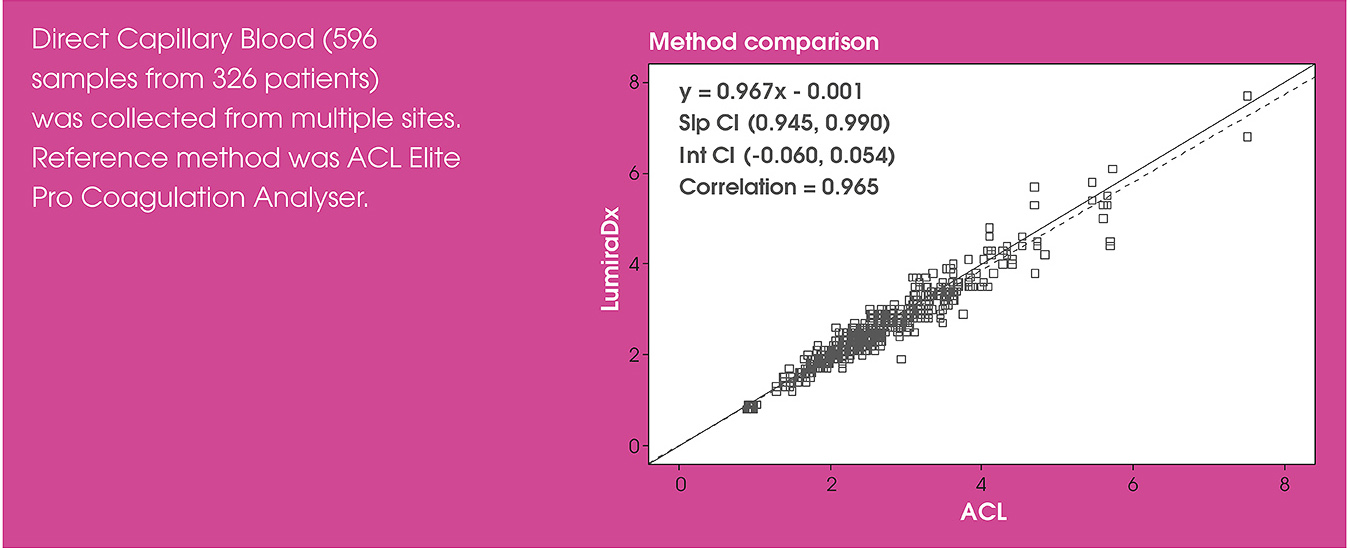
Test performance
In the OPTIMAL clinical trial, the LumiraDx INR Test correlated strongly with the Instrumentation Laboratory ACL Elite laboratory reference method. The trial found that the LumiraDx INR Test provided rapid and reliable INR results at the point of care1.
The LumiraDx INR Test is also highly precise. The following results represent the mean paired rep %CV for both direct and transfer tube application collected across multiple sites.
| Sample | n | Mean INR | Mean % CV |
|---|---|---|---|
| Direct application | 284 | 2.54 | 3.46 |
| Transfer Tube | 291 | 2.53 | 3.73 |
1. Tait RC, Hung A, Gardner RS. Performance of the LumiraDx Platform INR Test in an Anticoagulation Clinic Point-of-Care Setting Compared With an Established Laboratory Reference Method. Clin Appl Thromb Hemost. 2019.
More information about the LumiraDx INR Test:
Not all products are available in all countries and regions. Please check with your local LumiraDx sales representative or distributor for availability in specific markets. Not available in the USA.
BetterHealthExperiencesOutcomes
Supporting healthier lives, for individuals, communities and wider society.
Enabling responsive, personal relationships between patients and care teams.
Controlling and reducing costs to help ease pressure on healthcare budgets.
