CRP Test
The LumiraDx CRP (C-Reactive Protein) test is an easy to use, fast microfluidic immunoassay designed to rapidly quantify CRP levels in whole blood and plasma. Used with the LumiraDx Platform, the test delivers rapid, quantitative results in 4 minutes at the point of care.
Test benefits
The measurement of CRP provides information for the detection and evaluation of infection, tissue injury, inflammation disorders, and associated disease.
- Precise and lab comparable results
- Time to result: 4 minutes
- Sample types: Direct Fingerstick (or via Lithium Heparin transfer tube) venous (Lithium Heparin) or plasma (Lithium Heparin)
- Sample size: 20 μL
- Haematocrit correction for whole blood samples
- Storage at room temperature (2–30°C)
- Measuring range 5.0 - 250.0 mg/L
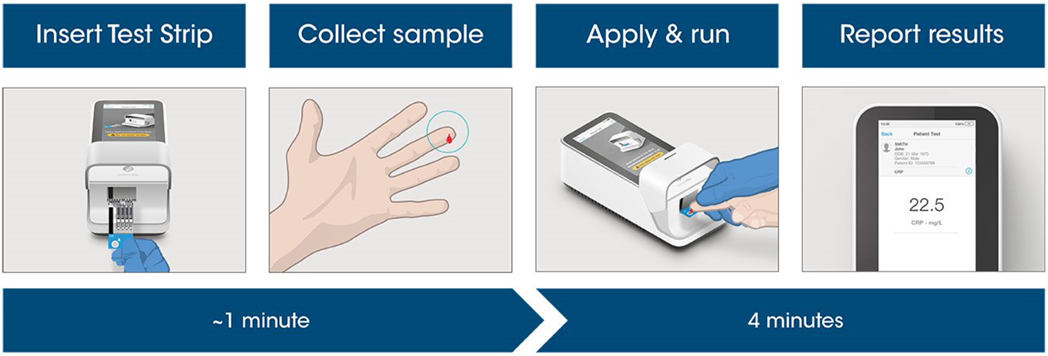
Test workflow
The Instrument and Test Strips are integrated with several quality control checks to ensure the Instrument and test are functioning correctly for every test run.
The workflow process is comprised of a simple sample collection with a fingerstick lancet followed by step-by-step guidance of the Instrument to report a patient result in 4 minutes from sample application.
Test performance
Method comparison
A comparison of 205 CRP measurements with the LumiraDx CRP test and the RCRP Flex® assay on the Siemens Dimension® Xpand® Plus Integrated Chemistry System was completed and analysed by Passing Bablok regression.
| Method comparison | |
|---|---|
| Slope | 1.00 |
| Intercept | -0.48 |
| r | 0.99 |
A precision study was carried out at 3 concentrations of CRP, each was tested in 1 run of 5 replicates per day, for five days across 3 sites. The results of the precision study are summarised below:
| CRP Concentration (mg/L) | Within Day Precision (%CV) | Between Day Precision (%CV) | Between Site Precision (%CV) | Total Precision (%CV) | n |
|---|---|---|---|---|---|
| 11.6 | 4.8 | 0.5 | 5.6 | 7.4 | 75 |
| 20.2 | 4.8 | 0.0 | 3.7 | 6.0 | 75 |
| 148.3 | 4.5 | 1.4 | 3.2 | 5.6 | 75 |
More information about the LumiraDx CRP test
Not all products are available in all countries and regions. Please check with your local LumiraDx sales representative or distributor for availability in specific markets.
BetterHealthExperiencesOutcomes
Supporting healthier lives, for individuals, communities and wider society.
Enabling responsive, personal relationships between patients and care teams.
Controlling and reducing costs to help ease pressure on healthcare budgets.