D-Dimer Test
The LumiraDx D-Dimer Test is an easy to use, fast microfluidic immunoassay designed to rapidly quantify D-Dimer levels in whole blood and plasma. It is the only direct fingerstick D-Dimer assay available today*, aiding healthcare professionals to exclude deep vein thrombosis (DVT) and pulmonary embolism (PE) in symptomatic patients with confidence** - in only 6 minutes.
The LumiraDx D-Dimer Test is an in vitro diagnostic test for the quantitative determination of D-Dimer in human capillary and venous whole blood and plasma samples (Sodium Citrate). The LumiraDx D-Dimer Test Strips are intended for use with the LumiraDx Instrument. It is an automated in vitro diagnostic test for near-patient testing to aid in the assessment and diagnosis of patients with suspected venous thromboembolism (VTE) such as deep vein thrombosis (DVT) and pulmonary embolism (PE).
The test can be used in conjunction with a clinical pre-test probability assessment model to exclude deep vein thrombosis (DVT) and pulmonary embolism (PE) disease in patients suspected of DVT or PE. The LumiraDx D-Dimer test is for Professional Use Only. For patients ≥18 years of age.
Test benefits
The LumiraDx D-Dimer Test improves efficiency in primary and secondary care settings by offering a rapid assessment of patients presenting with symptoms of deep vein thrombosis (DVT) and pulmonary embolism (PE).
- Clinical Cut-off: 500 µg/L FEU, 0.500 mg/L FEU
- Fast, accurate, quantitative results in just 6 minutes
- Direct fingerstick sampling
- Only 15µL sample volume required
- Room temperature Test Strip storage
- Precision: ≤11.1% CV
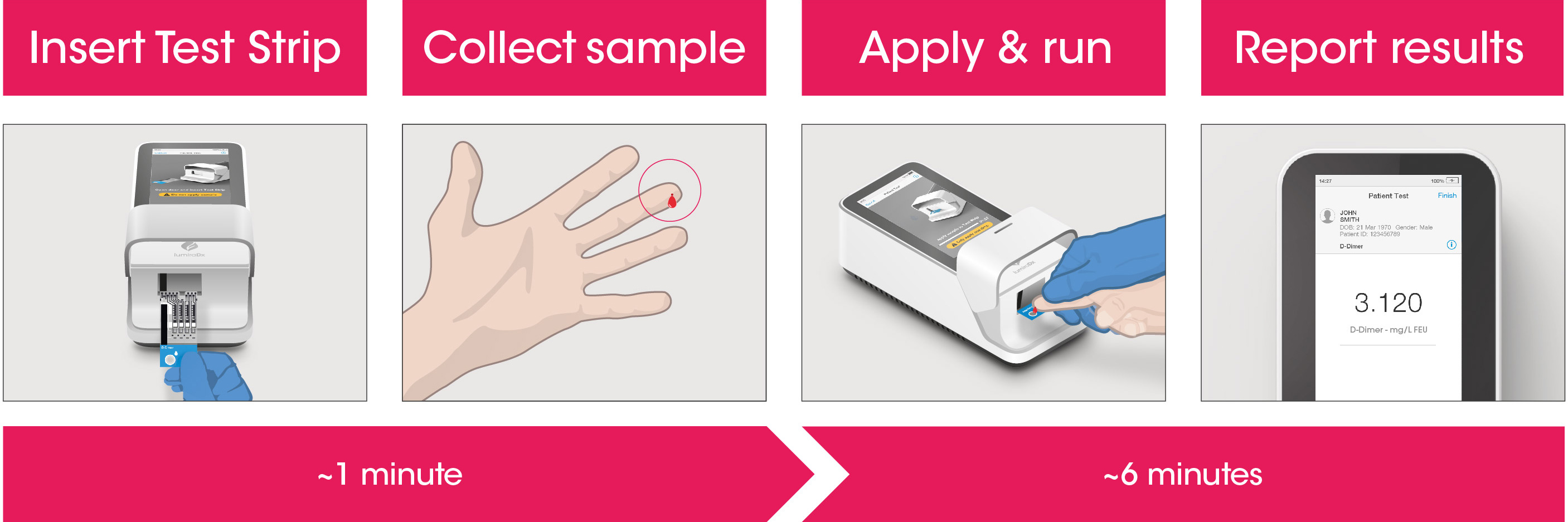
Test workflow
The Instrument and Test Strips are integrated with several quality control checks to ensure the Instrument and Test are functioning correctly for every test run.
The workflow process is comprised of a simple sample collection with a fingerstick lancet followed by step-by-step guidance of the Instrument to report a patient result in 6 minutes from sample application.
Test performance
Clinical Performance
A prospective clinical study was performed on 585 subjects where fresh samples (capillary blood, venous (blood citrated) and plasma (citrated)) were collected from patients presenting with symptoms of VTE (PE or DVT). Subjects also required an assessment with the Wells score and were classed as PTP ‘Likely’ or PTP ‘Unlikely’. Those with ‘Unlikely’ PTP categorization were further analysed using the LumiraDx D-Dimer test with 500 μg/L FEU (0.500 mg/L FEU) D-Dimer as cut-off. The corresponding sensitivity and negative predictive values (NPV) by sample matrix are listed below with corresponding Wilson Score 95% confidence intervals (CI).
| Estimate | Matrix | Patients with Suspected VTE |
|---|---|---|
| Unlikely PTP | ||
| Sensitivity % (95% CI) | Venous | 100.0% (74.1%-100.0%; n = 378) |
| Capillary Direct | 100.0% (72.2%-100.0%; n = 377) | |
| Plasma | 100.0% (74.1%-100.0%; n = 406) | |
| NPV % (95% CI) | Venous | 100.0% (98.3%-100.0%; n = 378) |
| Capillary Direct | 100.0% (98.1%-100.0%; n = 377) | |
| Plasma | 100.0% (98.1%-100.0%; n = 406) |
Method comparison
The method comparison was performed using plasma samples from patients (n = 327, range = 60 - 4515 μg/L FEU). A comparison of 1767 D-Dimer measurements with the LumiraDx D-Dimer Test to the VIDAS Exclusion II D-Dimer assay yielded the following statistics: Slope = 1.02, Intercept = 21, r = 0.92.
Precision
A precision study was carried out in citrated venous plasma on a protocol based on CLSI EP5-A3. The study was carried out with 3 levels of D-Dimer, each was tested in 2 runs of 2 replicates per day, for twenty days.
| D-Dimer concentration (μg/L FEU) | D-Dimer concentration (mg/L FEU) | Within run precision (% CV) | Within day precision (% CV) | Between day precision (% CV) | Total precision (% CV) | n |
|---|---|---|---|---|---|---|
| 291 | 0.291 | 9.8 | 11.1 | 0.0 | 11.1 | 80 |
| 552 | 0.552 | 9.4 | 9.4 | 2.5 | 9.7 | 80 |
| 1790 | 1.790 | 10.1 | 10.1 | 0.7 | 10.2 | 80 |
*As stated at time of publication - 24th May 2022
**In conjunction with a clinical pre-test probability assessment model.
For more information about the LumiraDx D-Dimer Test:
Not all products are available in all countries and regions. Please check with your local LumiraDx sales representative or distributor for availability in specific markets. Not available in the USA.
BetterHealthExperiencesOutcomes
Supporting healthier lives, for individuals, communities and wider society
Enabling responsive, personal relationships between patients and care teams.
Controlling and reducing costs to help ease pressure on healthcare budgets.