Fast Lab Solutions
Case Study
LumiraDx SARS-CoV-2 Antigen Test supports
COVID-19 prevention at Rotenburg District Hospital
The Rotenburg District Hospital (Kreiskrankenhaus Rotenburg an der Fulda)
Rotenburg District Hospital is a 183-bed general and standard care hospital with the departments of surgery (general, special visceral and trauma surgery, orthopedics, neurosurgery), internal medicine (gastroenterology, pulmonary and bronchial medicine, diabetology, rheumatology, oncology) and gynecology. The range of services is supplemented by cooperation with the Ambulatory Medical Care Center (MVZ: practices for surgery, neurology, internal medicine and primary care) and a radiology practice. Since the beginning of 2015, the hospital has been an academic teaching hospital of the Philipps University of Marburg.

Patients must receive necessary medical care at all times
Maintaining hospital operations with medical care for both inpatients and outpatients has become an ongoing challenge during the Coronavirus pandemic. To address this need, the Rotenburg District Hospital expanded its safety concept in October 2020 with the LumiraDx SARS-CoV-2 Antigen (point of care) Test. The test, in addition to other measurements, is supporting the early detection of COVID-19 cases and the prevention of outbreaks. Using LumiraDx’s fast antigen testing, as part of the hospital’s protocols, ensures that patients can always receive medical care, while maintaining the highest possible level of safety for staff and fellow patients.
Testing Procedure: Determining who is tested as part of the testing strategy
The overall strategy of Rotenburg District Hospital is to be able to offer “Covid-19-free” diagnostics, treatment and follow-up care to its approximately 17,000 patients annually. Patients with severe COVID-19 symptoms are treated in the hospital’s intensive care unit.
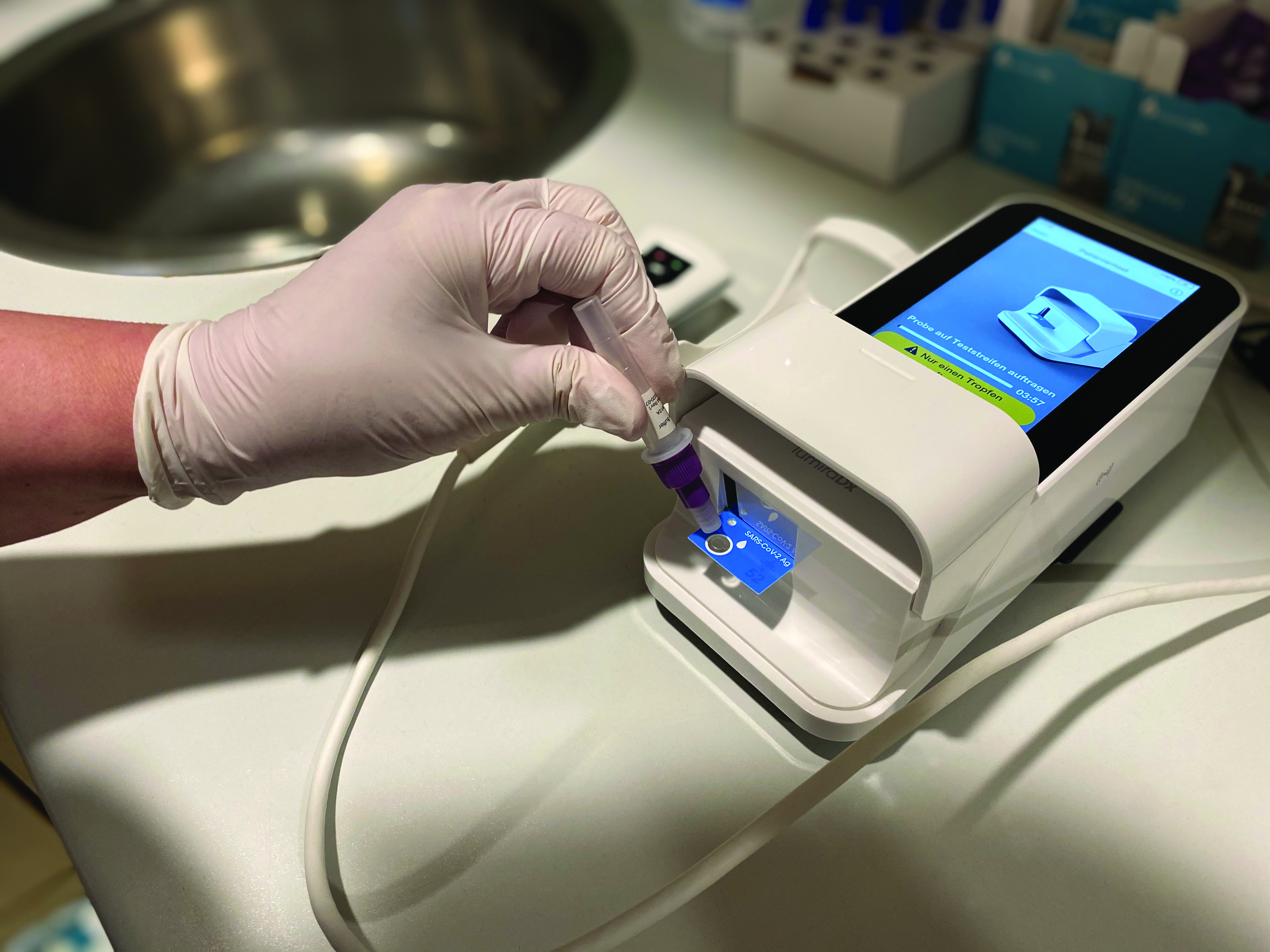
At the scheduled admission appointment, the test team performs pre-hospital SARS-CoV-2 antigen testing on every inpatient and outpatient at a dedicated testing station located at the main entrance of the hospital. To avoid delaying patient admission and treatment, a LumiraDx SARS-CoV-2 Antigen Test is performed, which displays a result in less than 12 minutes on the high-tech device’s screen after the prepared swab sample is applied. A negative rapid test result serves as an “admission ticket“ to the hospital.1 The tests are performed by trained lab- nursing staff (the “test team”).
Procedure in case of a negative result of therapid test
If the result of LumiraDx’s point of care test is negative, the patient is treated as if they had a negative PCR result.1 This procedure is covered by the national testing strategy and is supported both by the company’s own data on sensitivity as well as specificity and the data collected by the hospital laboratory.
“All patients, both outpatients and inpatients, are tested for COVID-19 virus infection before entering or being admitted to our hospital. Our employees also undergo regular SARSCoV-2 Antigen Testing. This allows us to ensure the highest possible level of safety for our patients and staff.”
Andreas Maus, Commercial Managing Director, Rotenburg District Hospital
Procedure in case of a positive result of the rapid test
If the SARS Cov-2 antigen test result is positive, the affected patient is either admitted to the COVID-19 ward as soon as possible or discharged to home quarantine or isolation if immediate inpatient or outpatient care is not necessary. In addition, a new swab is taken for confirmation of the result by PCR test. At the COVID-19 station, the patient is kept in protective isolation and treated until the PCR result is received. Once a negative PCR result is received, the patient can be de-isolated again for further treatment at the hospital. If the PCR is positive, the further course of action is decided according to the patient’s individual state of health and need for treatment.
Why did the Rotenburg District Hospital choose the LumiraDx SARS-CoV-2 Antigen Test?
In October 2020, the hospital had conducted a short test phase with the LumiraDx Platform and the SARS-CoV-2 Antigen Test. The test phase was led by the hospital management with support from the laboratory staff and the central patient admission. After evaluation of the experience and the results of comparative testing against PCR results obtained in the laboratory, the test was integrated into the clinical routine and has since been used as part of the hospital’s rapid testing concept. In addition to the testing of patients, regular testing is of course also available to all employees of the hospital. Another LumiraDx point of care device is made available in the laboratory for this purpose.
“The LumiraDx test system has helped us substantially during the pandemic to provide safe and smooth care to our patients and have safety measures in place for our staff. The handling is easy and it has been implemented into our daily routines.”
Karla Krause-Heid, Head Nurse and Human Resources Manager, Rotenburg District Hospital
High satisfaction among employees and patients
Staff and patients rate the speed and reliability of the test as very positive. Since a robust result is available quickly, the team is able to continue with patient care after a just a short waiting time. For the staff, the ease of use of the device is also of great importance when performing the test. This in turn means that only minimal training is required. The possibility of storing and transferring the results is also proving positive. In addition, a number of other tests for a wide range of medical indications can be tested with the point of care system in the future.
Conclusion
The introduction of rapid testing with the LumiraDx SARS-CoV-2 Antigen Test closes an inherent safety gap in the treatment of patients at the Rotenburg District Hospital and contributes to the maintenance of high-quality medicine under pandemic conditions. Initial results confirm the manufacturer’s claims and indicate high reliability and accuracy of the results.
1. The LumiraDx SARS-CoV-2 Ag Test result should not be used as the sole basis for treatment or case management decisions. To confirm the infection status a clinical correlation of the test result with the individual’s history and other diagnostic information is necessary.
The results shown here are specific to one health care facility and may differ from those achieved by other institutions.
Copyright pictures by Kreiskrankenhaus Rotenburg an der Fulda.
BetterHealthExperiencesOutcomes
Supporting healthier lives, for individuals, communities and wider society.
Enabling responsive, personal relationships between patients and care teams.
Controlling and reducing costs to help ease pressure on healthcare budgets.