Fast Lab Solutions Informationsportal
The LumiraDx SARS-CoV-2 Antigen Test demonstrated as more sensitive compared to SD Biosensor Standard Q COVID-19 Rapid Antigen Test in an independent evaluation on behalf of the Swiss Federal Office of Public Health
Original validation report was released by:
ADMed Microbiologie, Boucle de Cydalise 16, 2300 La Chaux-de-Fonds
Background
COVID-19 has significantly impacted global health and public life. The reliable detection of SARS-CoV-2 infections is of major importance in identifying and isolating positive cases, and also providing access to negative test results for normal life to resume. The LumiraDx SARS-CoV-2 Antigen (Ag) Test is a microfluidic immunofluorescence assay for the direct and qualitative detection of nucleocapsid protein (antigen) in nasal and nasopharyngeal swab specimens from individuals suspected of COVID-19 infection or from asymptomatic individuals. The described study aimed to validate the performance of the test in an independent, objective manner and was performed by “ADMed Microbiologie” as representative of the Swiss Society of Microbiology and on behalf of the Federal office of Public Health (FOPH), based in the “Bundesamt für Gesundheit” (BAG) in Switzerland.
Methods
300 patient nasopharyngeal samples were collected into 3 ml Amies buffer (n=200 negative, n=100 positive in RT-PCR for SARS-CoV-2 (Cobas 6800, Roche, E-gene considered for Ct values; Ct: 13-36). These were collected from routine patient RT-PCR testing and subsequently frozen. Specimens were thawed and analysed with the LumiraDx
SARS-CoV-2 Ag Test and the Standard Q COVID-19 Rapid Antigen Test from SD Biosensor/ Roche. Local laboratory procedures were used to prepare sample extracts. The resulting extracts were applied to the tests according to the manufacturer’s instructions for use. In addition, serial dilutions of SARS-CoV-2 positive samples were prepared and analysed with both Ag tests for comparison of detection limits.
Results
Results of the SARS-CoV-2 Ag Tests were compared to the RT-PCR Ct values from the Roche Cobas SARS-CoV-2 assay. At Ct 23, the LumiraDx SARS-CoV-2 Ag Test and the Standard Q COVID-19 Rapid Antigen Test from SD Biosensor/ Roche demonstrated 100% agreement with RT-PCR. At higher Ct values, which correlate with lower viral loads, the LumiraDx SARS-CoV-2 Antigen Test was more sensitive than the competitor (Figure 1). At Ct ≤ 29 the positive percent agreement (PPA) was 98,7% for the LumiraDx Ag Test and 92,0% for the SD Biosensor/ Roche Test (Table 1). The negative percent agreement was 99% for both methods compared in the study. Furthermore, the outstanding performance of the LumiraDx SARS-CoV-2 Ag Test was confirmed by the analysis of serially diluted samples, where this test detected samples diluted 2-titer higher than those detected by the SD Biosensor/ Roche Test.
| Ct Value (≤) | 23 | 29 | |
|---|---|---|---|
| PPA (%) | SD Biosensor/ Roche | 100 | 92.0 |
| LumiraDx | 100 | 98.7 |
Table 1: Performance of LumiraDx SARS-CoV-2 AG Test and Standard Q COVID-19 Rapid Antigen Test from SD Biosensor/ Roche at different Ct values.
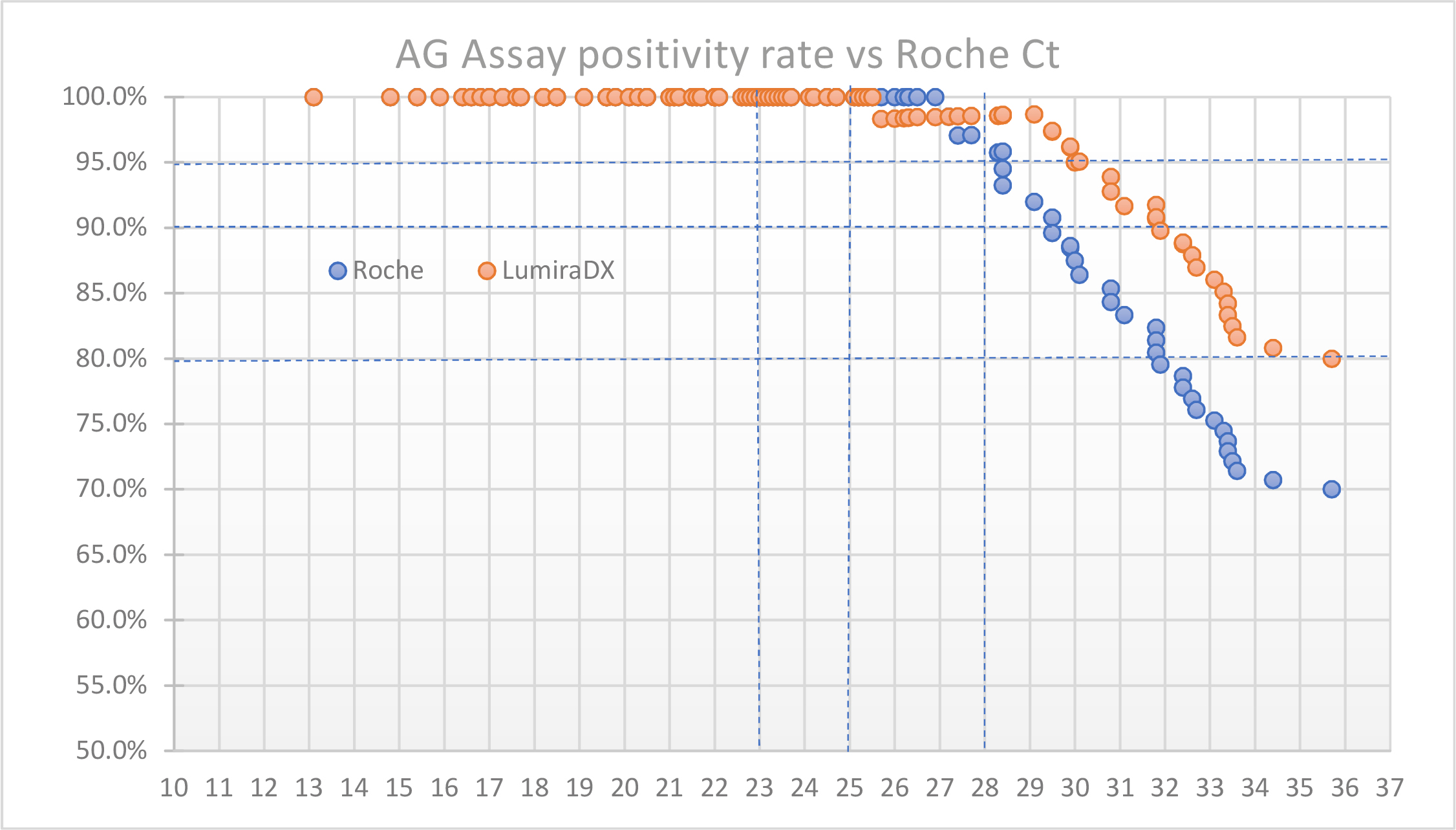
Figure 1: Positive percent agreement (PPA) dependent on Ct values. Up to a Ct of 25 (Cobas 6800, Roche) both tests performed equally. For Ct higher than 27 the LumiraDx SARS-CoV-2 Ag Test showed significantly higher PPA.
Conclusion
The LumiraDx SARS-CoV-2 Antigen Test passed the validation criteria of the Swiss Society of Microbiology and demonstrated higher sensitivity than the Standard Q COVID-19 Rapid Antigen Test (SD Biosensor/ Roche), especially when analysing samples with lower viral load. The LumiraDx SARS-CoV-2 Ag Test was consequently listed on the BAG website.
Reference
Report of BAG provided to LumiraDx.
Link to BAG List: https://www.bag.admin.ch/bag/de/home/medizin-und-forschung/heilmittel/covid-testung.html#-1047800939
Bessere GesundheitErfahrungenOutcomes
Wir unterstützen ein gesünderes Leben für Einzelpersonen, Communities und die Gesellschaft als Ganzes.
Wir ermöglichen responsive, persönliche Beziehungen zwischen Patienten und Versorgungsteams.
Wir kontrollieren und senken Kosten zur Entlastung des Budgets von medizinischen Einrichtungen.